The United States Food & Drug Administration has really been on my mind this last week. Let me set the background: I have a rare, progressive autoimmune disease that at this date has NOT ONE SINGLE DRUG that can directly treat it. Not one. There are drugs that target symptoms and the complications of my disease, systemic sclerosis, but none that can shut the disease down.
Over the last few days two alerts about new treatment developments for systemic sclerosis hit my newsfeed. One of the drugs, Certa Therapeutics’ FT011, is designed to treat chronic fibrosis and was just granted FDA Fast Track status. After a 12-week trial 60% of the systemic sclerosis patients had clinically significant improvement: I suspect that they are talking about lung function here. This is huge! This is the drug that I have been waiting for ever since I quit the anti-fibrotic drug OFEV last summer due to intolerable side effects. Fast track status means I may get this drug in another year or so. THIS IS HUGE, PEOPLE!!!! HUGE!!!

Just a couple days after the news about FT011 another news alert, even bigger news, came that Cabaletta Bio’s CABA-201 drug had been granted Orphan Drug status by the FDA. I’m not completely sure, but this seems to be a type of CAR T-cell therapy that would provide an immune system reset: a cure. Did you catch that? A CURE!!!!! The disease that I live with, systemic sclerosis, could be stopped dead in its track if this works. Orphan Drug status provides some financial incentives and helps in bringing the drug to market, but it doesn’t speed up the process like the Fast Track status will for FT011. Still, this is good news arriving all at once. I have a sense that momentum is building as these new, very sophisticated drug treatment strategies come to market based on specific molecular interventions in the patient.
Anyway, none of this going to happen overnight because the FDA approval process is very slow and painstaking. This is the way it needs to be to develop drugs safely. Drugs are first visualized based on knowledge of the regulation and complexities of biological systems. “Oh, that’s a good idea for a drug,” some scientist tells themself, thinking about a regulatory pathway in humans. They follow through on their idea and then see if it works in a specific science-based process that tests the drug in lab, animals and then finally humans to see if it will treat the disease/condition.

For example, one of my drugs is called Letairis. It is designed to treat pulmonary arterial hypertension, and it is an endothelin receptor antagonist. What the heck is that, you ask? As you might, because who in their everyday life would need to know about this stuff, right? Maybe you should skip this part if you are feeling sleepy… You’re still reading? Wow! I’m so impressed and grateful for your trust… Well, anyway, here is the very short version at the Midnight Knitter level of understanding: endothelin is a small protein produced by the cells lining the inside of my blood vessels that causes blood vessels to constrict. The drug that I take, Letairis, is a sneaky molecule that mimics endothelin; it binds to the receptor on the target site and blocks endothelin, keeping it from attaching to the receptor. The drug prevents my blood vessels from constricting and keeps my blood pressure in my lungs low. Yay!
Anyway, some scientist long ago had an idea that maybe blocking the action of endothelin would be a good way to control pulmonary hypertension. This idea was tested in the lab, then on animals, and then if all seemed okay it was tested on a very small group of humans, and then larger groups of humans. Data is collected and analyzed to look for the efficacy of the drug while also identifying all the possible side effects. There is a lot of risk/benefit analysis before the drug is released to market. After that more data is collected to hunt for bad side effects once the drug is being used in this much larger market.
I guess my point is, this is a long, long process with lots of safeguards along the way. The FDA is the agency that provides the scientific guard rails that protect me and every other drug consumer in the US from bad information, harmful drugs, and unscrupulous people who push pseudoscience treatments in order to make a buck. Thank you, FDA, for providing this essential service for me and every other American whether they appreciate it or not. I’m glad that you do this, even if it means I have to wait for my new drug that is slowly working its way through the process to come help me.

Today the United States Supreme Court heard arguments about the abortion drug mifepristone that centered around its approval by the FDA and the decision by that agency to allow it to be delivered by mail. I’m convinced that the issue has been raised solely because this drug is used for abortions, but the arguments brought before the court are suddenly extremely pertinent to me and my own situation.
I’m pretty sure that not one of the justices on the supreme court is qualified to make a judgement about the scientific process used to develop this drug and the analysis that was made about its safety. Just as I wouldn’t allow one of the large pharmaceutical companies to rule on a matter of law, I am alarmed that the courts are now going to second-guess a science-based agency.
I am also extremely concerned about the court deciding whether drugs can be sent to patients through the mail. It has to do with the ancient, mostly forgotten until now, laws on the books about drugs that can be used for abortions or contraception being delivered by mail.
Remember my drug Letairis? I need it to control my life-threatening pulmonary arterial hypertension that was gifted to me by my systemic sclerosis. This drug has a rigorous enrollment process and requires female patients to use two forms of birth control and to take a pregnancy test every month before they can get the next 30-day supply. Each month this drug is delivered to me by overnight express from a pharmacy in another state. This drug can harm an unborn child and may create the need for an abortion. Suddenly the arguments that were made today before the US Supreme Court threaten me and my access to medical care.
I sure hope that Certa’s FT011’s progress on the Fast Track isn’t affected by all of this. An upended FDA approval process could be disastrous for me and a lot of other people waiting for a new drug to arrive to save their butt.
Unintended consequences are a bitch.
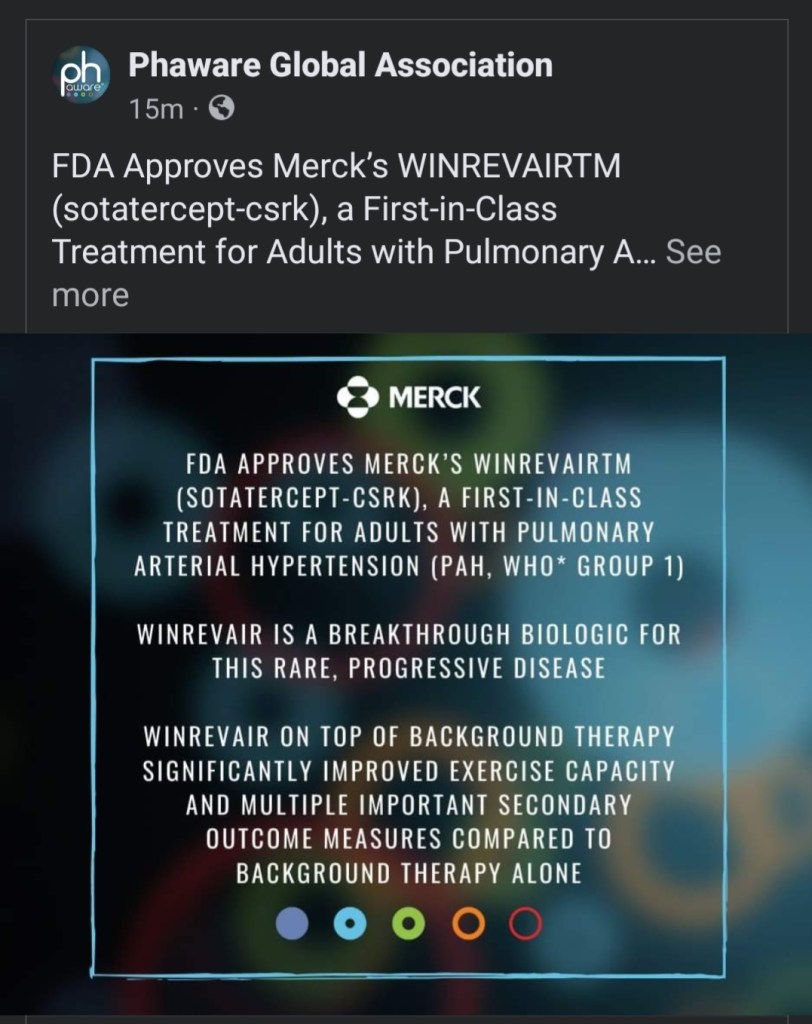
Update 3/27/2024: While I was writing this post yesterday, the FDA approved a new drug for pulmonary arterial hypertension (WHO Group 1). The relief and celebration in the online support communities this morning was pretty amazing. This is good news for me, too, as my PAH is in Group 1. Yay science!!

It is very charitable of you, Marilyn, to refer to SCOTUS’ “unintended consequences”: personally, I don’t believe that right-wing group of law-breakers gives a rat’s (_|_) about anything other than their personal attitudes and beliefs being promulgated.
I absolutely believe that some members of the court have been compromised and are now corrupt, but more than that, they are absolutely not qualified to evaluate the approvement process of a medication.
Of course you’re correct – how in the name of any of the gods could they POSSIBLY do it professionally ?!
This is an issue that you and your like-minded intelligent on-line mates should take up.
That’s why I decided to write about it today. If even a few people become aware of the FDA’s approval process and think twice about the absolute shitshow that would result if the courts start overturning science (and reality) based medical treatment options for women and everyone else, it will be worth the effot.
It will indeed ! I suppose you wouldn’t consider contacting the press ?
Like, send my blog post to Rachel Maddow? Yikes! I don’t think that I write that well. 🙂
Exactly like the wonderful Rachel Maddow !!! I think you write better than “that well”, Marilyn; and this is the kind of issue that she would revel in picking up !!!
Oh, she is pretty busy with the current political shitshow, but I thank you. The irony here is… I have a biology degree because if you majored in a science area you didn’t have to write any freaking term papers!!! Plus, I love science/biology.
Yesyes, I know all that. But you’re not seeing this in the correct light, Marilyn: THIS IS VERY POLITICAL !! Maddow would lap it up !!!
Well, you know Rachel only is on once a week now. I’m thinking that this issue is one of tooooo many scary things going on right now. I will write all my congressmen.
That’s exciting news regarding those 2 drugs. Hooray for science! And you’re right, the Supreme Court is not qualified to rule on drug safety. They’re not scientists.
Yay for science, indeed!! I agree with you; the court is not qualified to rule on drug safety. If they do not leave this in the hands of the FDA we are all going to be in trouble.
Thank goodness that you brain seems unimpeded
Great post and I agree with you 100%. I do not know about anyone else, but I have had enough of the political nonsense in the country. I have never been political, but I have strong beliefs.
Off-topic, I love your plant in one of the pictures. It is absolutely beautiful. I also LOVE your cats. What kind of cat is, Mateo? I was thinking Maine coon, as we have one who is at least half and they have some of the same features.
I used to pride myself on not being political, but that was before there was so much opposition to allowing people to obtain no-fault health care insurance. After that I was suddenly political and switched parties. I was also teaching in a school with a complicated student body filled with American citizen children who were discriminated against (black), students with dubious legal status, and refugee students from all over the world. I made me rethink things.
Both of my cats came from a shelter and were rescues during the kitten season. I have wondered about Mateo a lot, as that tail is insane! I think he is part Maine coon, as he has the ruff, the fur over his shoulders and front legs is shorter and smooth, and he trills and chirps a lot. Like, a lot! His rear end is also higher, which is evidently a Maine coon trait. He’s pretty small for that breed, however.
Yay for science! I am so glad these promising new medications are on the horizon! Hoping for no Supreme Court malarky to mess up you getting your much needed pharmaceuticals as well!
I sounds like the Supreme Court will not rule in their favor, but just the possibility that the drug approval process could be upended by legal second-guessing is just shocking and should not be allowed, especially when it is based on the purpose of the drug instead of the process itself.
What exciting news!! I know the drug approval process is frustratingly long. Is it possible that one of your doctors could get you into a trial? Early access, etc. Just a thought.
I agree wholeheartedly that judges/lawyers should not be practicing medicine and doctors should not be practicing law. If the court has a medical issue to rule on, they should listen to the professional opinions of a number of physicians and medical researchers. Otherwise their respective medical and legal degrees mean nothing.
I’ve noticed a glut of new drugs being advertised on tv in recent weeks. Almost one a day, it seems. Maybe that bodes well …
My doctors talked to me about a trial, and I looked at the ones at National Jewish, but as it turns out I don’t fit the enrollment criteria as I have too many complications going. Sigh. Still, it is really encouraging that these drugs are on the way. Another new drug was also approved recently, a monoclonal antibody preventive that can be given to people with compromised immune systems to protect from Covid: I already contacted my doctor to see if I qualify. Yay science!!